
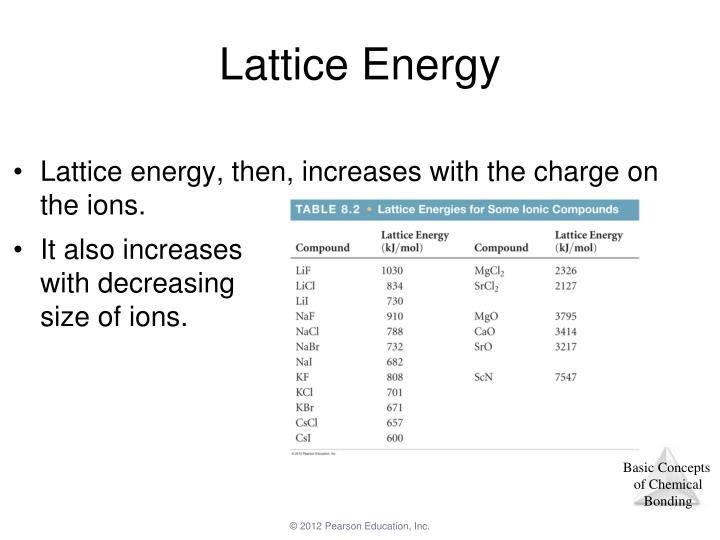
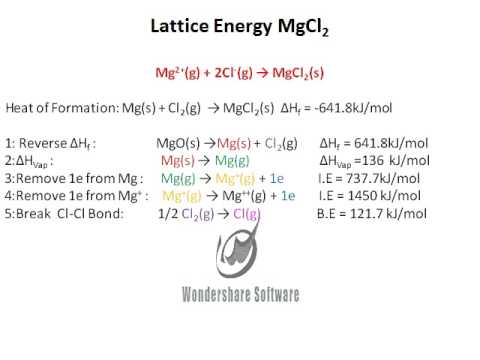
In both cases, a larger magnitude for lattice energy indicates a more stable ionic compound. Thus, if you are looking up lattice energies in another reference, be certain to check which definition is being used. Hint:- charge of ions and lattice enthalpy. Give plausible reason for this observation. Which of the following is a correct order of lattice energy Ans. The first point explains why MgO has a higher lattice energy than NaF. Thus point 2 addresses point 2 in your question, K is bigger than Li, hence the separation is bigger in KF than LiF, hence KF has a lower lattice energy than LiF. Small separation means high lattice energy. Some texts use the equivalent but opposite convention, defining lattice energy as the energy released when separate ions combine to form a lattice and giving negative (exothermic) values. But the melting point of NaF is 992C and that of MgO is 2642C. 2 unpaired electrons for sulfur atom (Table 8.1) 0 unpaired electrons for sulfide ion. High charges on the ions mean high lattice energy. Note that we are using the convention where the ionic solid is separated into ions, so our lattice energies will be endothermic (positive values). The bond energy for a diatomic molecule, D X–Y, is defined as the standard enthalpy change for the endothermic reaction: The energy required to break a specific covalent bond in one mole of gaseous molecules is called the bond energy or the bond dissociation energy. The bond length is the internuclear distance at which the lowest potential energy is achieved.
The potential energy of two separate hydrogen atoms (right) decreases as they approach each other, and the single electrons on each atom are shared to form a covalent bond.


 0 kommentar(er)
0 kommentar(er)
